CPT 2013 added to the table (p. 177 professional edition) "Conversion of existing system to bi-ventricular system (addition of LV lead and removal of current generator with insertion of new pulse generator with bi-ventricular pacing capabilities 33225 + 33228 or 33229 (pacemaker) 33225+33263 or 33264 (ICD)"
Full Answer
Is CPT code 99070 a valid and Billable code?
Oct 03, 2018 · A56341 - Billing and Coding: Implantable Automatic Defibrillators LCDs L33271 - Biventricular Pacing/Cardiac Resynchronization Therapy
What is the CPT code for IUD removal and insertion?
Oct 01, 2015 · Please refer to the Local Coverage Article: Billing and Coding: Biventricular Pacing/Cardiac Resynchronization Therapy (A57634) for utilization guidelines that apply to the reasonable and necessary provisions outlined in this LCD. Sources of Information. First Coast Service Options, Inc. reference LCD number L32811.
What is a Current Procedural Terminology (CPT) billing code?
Jan 26, 2015 · I have a table in my cpt book (professional edition, pg 187) that specifies this procedure. The codes listed are :33225 +33228 or 33229. I only see 2 leads documented, so I would code 33225+33228. And, I agree, this is more involved than just the requirements of 33214.
What is an ICD with biventricular pacemaker implant?
CPT‡ CODE DESCRIPTION STATUS INDICATOR APC NATIONAL MEDICARE RATE GENERATOR IMPLANT 33212 Insertion of pacemaker pulse generator only; with existing single lead J1 5222 $7,641 33213 Insertion of pacemaker pulse generator only; with existing dual leads J1 5223 $10,251 RELOCATION OF SKIN POCKET 33222 Relocation of skin pocket for pacemaker T ...

What is the CPT code for biventricular ICD implant?
CPT® 33249, Under Pacemaker or Implantable Defibrillator Procedures.
What is a biventricular ICD?
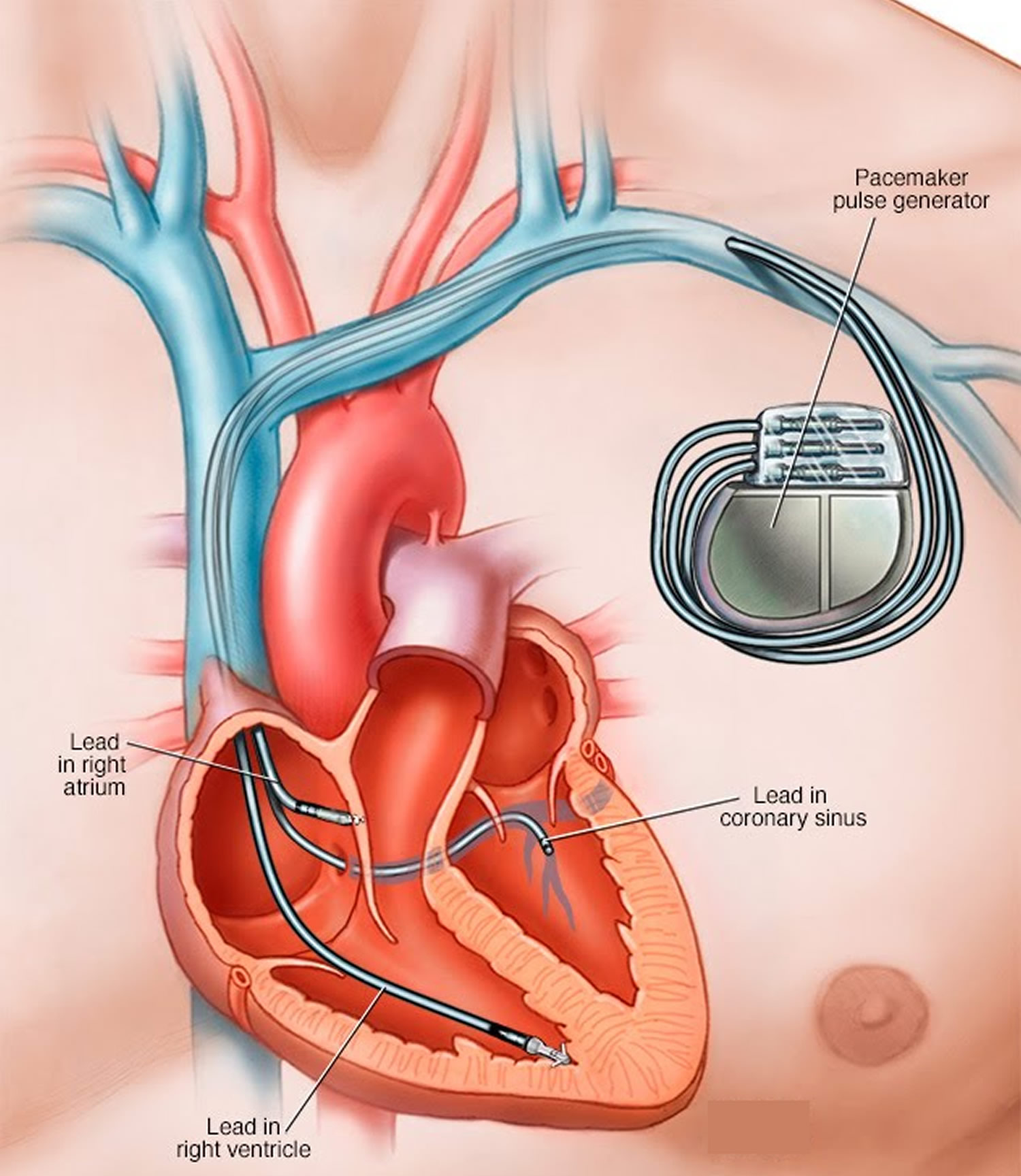
A biventricular pacemaker and ICD is a small, lightweight device powered by batteries. This device helps keep your heart pumping normally. It also protects you from dangerous heart rhythms. Read on to learn more about this device and how it works.
What is the CPT code for biventricular ICD generator change?
IC: Use CPT® code 33224: Insertion of pacing electrode, cardiac venous system, for left ventricular pacing, with attachment to previously placed pacemaker or implantable defibrillator pulse generator (includes revision of pocket, removal, insertion, and/or replacement of existing generator).Sep 29, 2016
What is procedure code 33249?
33249. INSERTION OR REPLACEMENT OF PERMANENT IMPLANTABLE DEFIBRILLATOR SYSTEM, WITH TRANSVENOUS LEAD(S), SINGLE OR DUAL CHAMBER. 33262. REMOVAL OF IMPLANTABLE DEFIBRILLATOR PULSE GENERATOR WITH REPLACEMENT OF IMPLANTABLE DEFIBRILLATOR PULSE GENERATOR; SINGLE LEAD SYSTEM.
Is a biventricular ICD a dual chamber?
Pacemakers that pace both the right atrium and right ventricle of the heart and require 2 pacing leads are called "dual-chamber" pacemakers. Pacemakers that pace the right and left ventricles are called "biventricular" pacemakers.
Does a BIV ICD pace?
Currently, doctors use an ICD to prevent these dangerous rhythms. The device works by detecting such a rhythm and shocking the heart back to normal. These devices can combine biventricular pacing with anti-tachycardia pacing (to slow down the heart rate) and internal defibrillators (ICDs) to deliver shocks as needed.Aug 24, 2020
What CPT code is 33208?
Group 1CodeDescription33207INSERTION OF NEW OR REPLACEMENT OF PERMANENT PACEMAKER WITH TRANSVENOUS ELECTRODE(S); VENTRICULAR33208INSERTION OF NEW OR REPLACEMENT OF PERMANENT PACEMAKER WITH TRANSVENOUS ELECTRODE(S); ATRIAL AND VENTRICULAR1 more row
What is the CPT code for subcutaneous ICD generator change?
A subcutaneous implantable defibrillator (S-ICD) consists of a subcutaneous or submuscular pulse generator and a single subcutaneous lead. S-ICD insertion (33270) is payable under the Medicare Physician Fee Schedule and the Outpatient Prospective Payment System.
What does AICD stand for in medical terms?
The automatic implantable cardioverter-defibrillator (AICD) is a device designed to monitor the heartbeat. This device can deliver an electrical impulse or shock to the heart when it senses a life-threatening change in the heart's rhythm.
What is CPT code 33225?
Pacemaker or Implantable Defibrillator ProceduresCPT® 33225, Under Pacemaker or Implantable Defibrillator Procedures. The Current Procedural Terminology (CPT®) code 33225 as maintained by American Medical Association, is a medical procedural code under the range - Pacemaker or Implantable Defibrillator Procedures.
What does CPT code 93296 mean?
Coding Clarification: CPT code 93296 refers to pacemaker systems in addition to implantable cardiac defibrillator systems in. its descriptor.Jun 9, 2021
Does CPT 33249 need modifier?
Q0 - Append this modifier on a category B IDE code (e.g. CPT ® 33249- Insertion or replacement of permanent implantable defibrillator system, with transvenous lead(s), single or dual chamber) if data is submitted to an FDA -approved category B IDE clinical trial, a trial under the CMS Clinical Trial Policy, or a ...Dec 20, 2019
What is ICD coding?
The Cardiac Pacemakers, Implantable Cardioverter Defibrillators (ICD), Cardiac Resynchronization Therapy and Implantable/Insertable Cardiac Monitors (ICM) Coding Guide is intended to provide reimbursement educational information tied to use of these products when used consistently with the products' labeling. This guide includes information regarding coverage, coding and reimbursement, as well as general information regarding appealing denied claims and supporting documentation.
What is the CPT code for remote cardiac monitoring?
Effective January 1, 2020, the code for the technical component of remote monitoring for Implantable Cardiovascular Physiologic Monitoring Systems and Implantable/Insertable Cardiac Monitors (ICMs), CPT Code 93299, will be deleted. The Centers for Medicare & Medicaid Services (CMS) created a new G-code, G2066, to report this service. G2066 can be reported by physicians and outpatient hospitals. G2066 will continue to be carrier-priced, as 93299 was, and the description of the code will be the same. See pages 49 and 53 for more information.
What is the add on code for CRT?
Add-on code 33225 can be performed when medically appropriate with the primary service/procedure codes listed below. Add-on codes may not be reported as a stand-alone and must be billed when performed in conjunction with the primary service or procedure. Add-on codes qualify for separate payment for physicians and are not subject to the Physician Multiple Payment Reduction Rule.
When was CRT D approved?
In September 2010, the FDA approved a new indication for 3 cardiac resynchronization therapy defibrillators (CRT-D) used to treat certain heart failure patients. The new use is for patients with left bundle branch block, which occurs when there is delayed activation and contraction of the left ventricle.
Is Aetna approved for biventricular pacemakers?
Aetna considers Food and Drug Administration (FDA)-approved biventricular pacemakers ( cardiac resynchronization therapy) medically necessary for the treatment of members with congestive heart failure (CHF) who are in sinus rhythm when either of the following criteria is met (A or B):
What percentage of CHF patients do not respond to CRT?
Reddy and colleagues (2017) noted that a total of 30 % to 40 % of patients with CHF eligible for CRT either do not respond to conventional CRT or remain untreated due to an inability or impediment to coronary sinus (CS) lead implantation. The WiSE-CRT system (EBR Systems, Sunnyvale, CA) was developed to address this at-risk patient population by performing bi-ventricular pacing via a wireless LV endocardial pacing electrode. The SELECT-LV (Safety and Performance of Electrodes implanted in the Left Ventricle) study is a prospective, multi-center, non-randomized trial evaluating the safety and effectiveness of the WiSE-CRT system. A total of 35 patients indicated for CRT who had "failed" conventional CRT underwent implantation of an LV endocardial pacing electrode and a subcutaneous pulse generator. System performance, clinical efficacy, and safety events were assessed out to 6 months post-implant. The procedure was successful in 97.1 % (n = 34) of attempted implants. The most common indications for endocardial LV pacing were difficult CS anatomy (n = 12), failure to respond to conventional CRT (n = 10), and a high CS pacing threshold or phrenic nerve capture (n = 5). The primary performance end-point, bi-ventricular pacing on the 12-lead electrocardiogram (EKG) at 1 month, was achieved in 33 of 34 patients. A total of 28 patients (84.8 %) had improvement in the clinical composite score at 6 months, and 21 (66 %) demonstrated a positive echocardiographic CRT response (greater than or equal to 5 % absolute increase in LVEF). There were no peri-cardial effusions, but serious procedure/device-related events occurred in 3 patients (8.6 %) within 24 hours, and 8 patients (22.9 %) between 24 hours and 1 month. The authors concluded that the SELECT-LV study demonstrated the clinical feasibility of the WiSE-CRT system, and provided clinical benefits to a majority of patients within an otherwise "failed" CRT population. This clinical trial is still ongoing, but not recruiting subjects (Last updated September 27, 2016).
Is myocardial fibrosis a predictor of aortic stenosis
Arangalage and colleagues (2016) stated that myocardial fibrosis has been proposed as an outcome predictor in asymptomatic patients with severe aortic stenosis (AS) that may lead to consider prophylactic surgery. It can be detected using MRI but its widespread use is limited and development of substitute biomarkers is highly desirable. These researchers analyzed the determinants and prognostic value of galectin-3, one promising biomarker linked to myocardial fibrosis. Patients with at least mild degenerative AS enrolled between 2006 and 2013 in 2 ongoing studies, COFRASA/GENERAC (COhorte Française de Rétrécissement Aortique du Sujet Agé/GENEtique du Rétrécissement Aortique), aiming at assessing the determinants of AS occurrence and progression, constituted the study population. These investigators prospectively enrolled 583 patients. The mean galectin-3 value was 14.3 ± 5.6 ng/ml. There was no association between galectin-3 and functional status (p = 0.55) or AS severity (p = 0.58). Independent determinants of galectin-3 were age (p = 0.0008), female gender (p = 0.04), hypertension (p = 0.002), diabetes (p = 0.02), reduced LVEF (p = 0.01), diastolic dysfunction (E/e', p = 0.02) and creatinine clearance (p < 0.0001). Among 330 asymptomatic patients at baseline, galectin-3 was neither predictive of outcome in univariate analysis (p = 0.73), nor after adjustment for age, gender, rhythm, creatinine clearance and AS severity (p = 0.66). The authors concluded that in a prospective cohort of patients with a wide range of AS severity, galectin-3 was not associated with AS severity or functional status. Main determinants of galectin-3 were age, hypertension and renal function. They stated that galectin-3 did not provide prognostic information on the occurrence of AS-related events; and that these findings did not support the use of galectin-3 in the decision-making process of asymptomatic patients with AS.
What is DCM in heart transplant?
Wojciechowska and colleagues (2017) noted that dilated cardiomyopathy (DCM) is the 3rd cause of HF and the most frequent cause of heart transplantation (HT). The value of biomarkers in prognostic stratification may be important in identifying patients for more advanced treatment. Assessment of serum galectin-3 (Gal-3) and ST2 as biomarkers of unfavorable outcome (death and combined end-point: HT or death or left ventricular assist device [LVAD] implantation) in stable DCM patients. A total of 107 DCM patients aged 39 to 56 years were included into the study and followed-up for a mean of 4.8 years; Gal-3 and ST2 concentrations were measured using ELISA tests. Clinical data, treatment, laboratory parameters, NT-proBNP, Gal-3 and ST2 measured at time of inclusion were assessed as risk factors for reaching the study end-points using log rank test and Cox proportional-hazards model. During follow-up, 27 patients died, 40 achieved combined end-point; ROC curves indicated cut-off value of ST2 -- 17.53 ng/ml, AUC-0.65 (0.53 to 0.76) and of NT-proBNP -- 669 pg/ml, AUC 0.61 (0.50 to 0.73) for prediction of death. In multi-variate analysis, ST2 was predictor of death (HR per unit increase in log ST2 2.705, 95 % CI: 1.324 to 5.528, p = 0.006) and combined end-point (HR per unit increase in log ST2 2.753, 95 % CI: 1.542 to 4.914, p < 0.001). The authors concluded that NT-proBNP was predictive variable only for death in multi-variate analysis; Gal-3 concentration was not associated with adverse outcome; ST2 but not Gal-3 may be useful for predicting adverse outcome in stable DCM patients.
Can HF patients continue CRT?
Gopinathannair and associates (2018) stated that many patients with HF continue CRT after continuous flow LVAD (CF-LVAD) implant. In a multi-center, non-randomized, observational study, these investigators reported the first study that examined the impact of CRT on clinical outcomes in CF-LVAD patients. Analysis was carried out on 488 patients (58 ± 13 years, 81 % men) with an implantable cardioverter defibrillator (ICD) (n = 223) or CRT-D (n = 265) who underwent CF-LVAD implantation at 5 centers from 2007 to 2015. Effects of CRT on mortality, hospitalizations, and ventricular arrhythmia incidence were compared against CF-LVAD patients with an ICD alone. Baseline differences were noted between the 2 groups in age (60 ± 12 years versus 55 ± 14 years, p < 0.001) and QRS duration (159 ± 29 ms versus 126 ± 34 ms, p = 0.001). Median biventricular pacing in the CRT group was 96 %. During a median follow-up of 478 days, Kaplan-Meier analysis showed no difference in survival between groups (log rank p = 0.28). Multi-variate Cox regression demonstrated no survival benefit with type of device (ICD versus CRT-D; p = 0.16), whereas use of amiodarone was associated with increased mortality (HR 1.77, 95 % CI: 1.1 to 2.8, p = 0.01). No differences were observed between CRT and ICD groups in all-cause (p = 0.06) and HF (p = 0.9) hospitalizations, ventricular arrhythmia incidence (43 % versus 39 %, p = 0.3), or ICD shocks (35 % versus 29 %, p = 0.2). During follow-up, 69 (26 %) patients underwent pulse generator replacement in the CRT-D group compared with 36 (15.5 %) in the ICD group (p = 0.003). The authors concluded that in this large, multi-center CF-LVAD cohort, continued CRT was not associated with improved survival, hospitalizations, incidence of ventricular arrhythmia and ICD therapies, and was related to a significantly higher number of pulse generator changes. These researchers stated that these findings supported discontinuing biventricular pacing following CF‐LVAD implant to preserve battery life and reduce generator replacements. They stated that large, prospective, randomized studies are needed to determine the role of CRT on ventricular remodeling and clinical outcomes in CF‐LVAD patients.
What is the NYHA classification of HF?
The NYHA classification of HF is a 4-tier system that categorizes patients based on subjective impression of the degree of functional compromise. The 4 NYHA functional classes are as follows:
Popular Posts:
- 1. icd 10 code for back pain fall
- 2. 2019 icd 10 code for 2ndry osteoarthritis right hip
- 3. icd 10 code for hx of multiple sclerosis
- 4. icd 10 dx code for carpal tunnel syndrom
- 5. icd 10 code for history of ileostomy reversal
- 6. icd-10 code for kidney injury unspecified
- 7. icd 10 code for abrasion to left arm
- 8. icd 10 cm code for cyst of left humerus
- 9. icd 10 code for opioid use
- 10. what is the correct icd 10 code for right subcapsular kidney hematoma