Encounter for screening for human immunodeficiency virus [HIV] Z11.4 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2019 edition of ICD-10-CM Z11.4 became effective on October 1, 2018.
What are the new ICD 10 codes?
ICD-10 QUICK REFERENCE: LABORATORY PREVENTATIVE SCREENING [Type text] [Type text] updated 3/10/16 Human Immunodeficiency Virus (HIV) Screening HIV Antibody No Increased Risk Factors Z11.4 Increased Risk Z11.4 & Z72.89, Z72.51, Z72.52 or Z72.53 Pregnant Women Z11.4 + one of the following: Z34.00-Z34.03, Z34.80-Z34-83,
What is the ICD 10 diagnosis code for?
2022 ICD-10-CM Diagnosis Code B20 Human immunodeficiency virus [HIV] disease 2016 2017 2018 2019 2020 2021 2022 Billable/Specific Code B20 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2022 edition of ICD-10-CM B20 became effective on October 1, 2021.
What is the CPT code for HIV screening?
Source: National Coverage Determinations Coding Policy Manual and Change Report (ICD-10-CM) April 2018 Effective May 1, 2018 Medicare Limited Coverage Tests. HIV Testing (Diagnosis) National Coverage Determination. CPT Codes: Code Description 86689 . Qualitative or semiquantitative immunoassays performed by multiple step methods; HTLV or HIV
What does ICD 10 do you use for EKG screening?
• CPT codes 1. Test product 86701 HIV-1 or HIV-2 antibody test 2. Test administration 36415 collection of venous blood by venipuncture 3. Office service 99211–99215 appropriate office visit code from the office or other outpatient services code series for an established patient based upon the key components performed or 2
See more
HIV antigen/antibody, combination assay, screening : S3645: HIV-1 antibody testing of oral mucosal transudate: ICD-10 codes covered if selection criteria are met: B20: Human immunodeficiency virus [HIV] disease: Z11.4: Encounter for screening for human immunodeficiency virus [HIV] Z20.6: Contact with and (suspected) exposure to human …
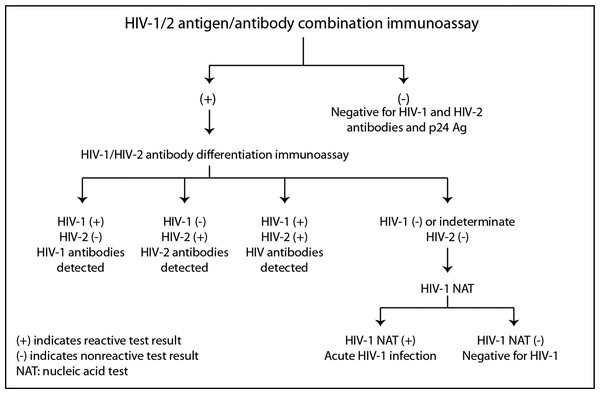
What is the ICD-10 code for HIV screening?
What is the CPT code for HIV screening?
What ICD-10 covers HIV?
What is ICD-10 code Z21?
What is code Z71 7?
What is procedure code 86803?
Is B20 always coded first?
What is the late phase of HIV?
One or more indicator diseases, depending on laboratory evidence of hiv infection (cdc); late phase of hiv infection characterized by marked suppression of immune function resulting in opportunistic infections, neoplasms, and other systemic symptoms (niaid). rheumatoid arthritis ( M05.-)
What is immunodeficiency syndrome?
Clinical Information. A disease caused by human immunodeficiency virus (hiv). People with acquired immunodeficiency syndrome are at an increased risk for developing certain cancers and for infections that usually occur only in individuals with a weak immune system.
What is CD4 positive?
An acquired defect of cellular immunity associated with infection by the human immunodeficiency virus (hiv), a cd4-positive t-lymphocyte count under 200 cells/microliter or less than 14% of total lymphocytes, and increased susceptibility to opportunistic infections and malignant neoplasms.
What is a type 1 exclude note?
A type 1 excludes note indicates that the code excluded should never be used at the same time as B20. A type 1 excludes note is for used for when two conditions cannot occur together, such as a congenital form versus an acquired form of the same condition.
Does Aetna test for HIV?
Aetna considers human immunodeficiency virus ( HIV) testing medically necessary for screening persons for HIV infection, according to the recommendations of the U.S. Preventive Services Task Force and the Centers for Disease Control and Prevention.
Is the Reveal G4 antibody rapid test FDA approved?
Rossetti and associates (2020) stated that the Reveal G4 antibody rapid test is FDA-approved for HIV-1 detection using the versions LAB S/P and point-of-care (POC) in CLIA-moderate complexity settings with serum/plasma and whole blood, respectively. The same Reveal tests are CE-marked for HIV-1 and HIV-2 detection in laboratory and POC settings. These investigators compared the performance of G4 LAB S/P with plasma and POC with whole blood (blood) for detecting early and established HIV-1/HIV-2 infections. Matched well-characterized plasma and simulated blood were used to evaluate: sensitivity in 104 HIV-1 and 55 HIV-2 established infections, specificity in 49 HIV-negative, and reactivity in early HIV-1 infection in a performance panel (n = 38) and 18 plasma panels from sero-converters (SCs, n = 183). Median number of days after first RNA-positive was calculated for 13 SCs. Impact of viral suppression (VS) was evaluated in 3 SCs receiving early anti-retroviral therapy (ART). Sensitivity was 100 % for HIV-1 and 98.18 % for HIV-2, while specificity was 100 %. All 38 plasma and blood become reactive by Fiebig stage V. Of 18 SCs, 10 had similar reactivity in plasma/blood, 7 showed delayed reactivity in blood, and 1 was non-reactive in plasma/blood. The median days for a G4-reactive after first RNA-positive was 13 for plasma and 14 for blood. Long-term VS had no impact on G4 reactivity. The authors concluded that overall reactivity in early HIV-1 infections was delayed by 1 day in blood compared to plasma. These researchers stated that if FDA-approved for POC settings, the G4 POC is a fast sensitive screening tool for HIV-1/HIV-2-specific IgG even during VS.
What is the code for a confirmed diagnosis?
For a confirmed diagnosis, assign code U07.1, COVID-19. This is an exception to the hospital inpatient guideline Section II, H. In this context, “confirmation” does not require documentation of the type of test performed; the provider’s documentation that the individual has COVID-19 is sufficient.
What is A00-B99?
Chapter 1: Certain Infectious and Parasitic Diseases (A00-B99) g. Coronavirus Infections. Code only a confirmed diagnosis of the 2019 novel coronavirus disease (COVID-19) as documented by the provider, documentation of a positive COVID-19 test result, or a presumptive positive COVID-19 test result.
Does confirmation require documentation?
In this context, “confirmation” does not require documentation of the type of test performed; the provider’s documentation that the individual has COVID-19 is sufficient. Presumptive positive COVID-19 test results should be coded as confirmed.
Popular Posts:
- 1. icd 10 code for bilateral subdural hematoma
- 2. icd 10 code for right side acute chf
- 3. icd 10 code for hypogastric pain
- 4. icd 10 2017 medical billing code for primarymalignancy of the left tonsil
- 5. icd 10 code for recurrent croup
- 6. icd 10 code for perisplenic fluid collection
- 7. icd-10 code for anoxia due to high altitude, initial encounter
- 8. icd 10 code for explore appendagitis
- 9. what is the icd 10 code for hodgkin's sarcoma of thoracic lymph nodes
- 10. icd 10 code for cpain