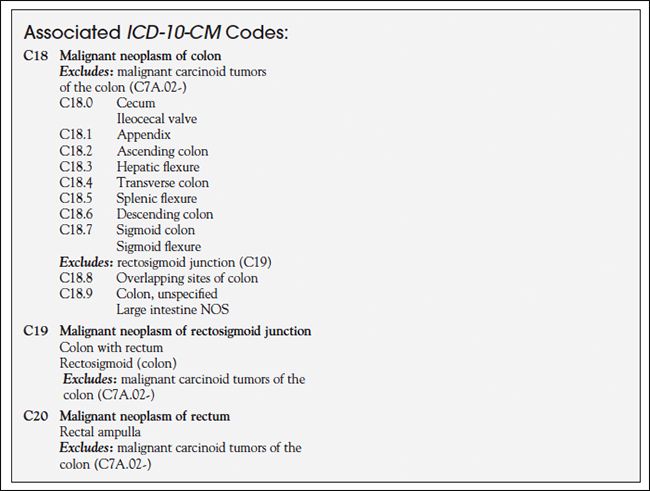
What is the ICD 10 DX code for Nexplanon implant status?
Oct 01, 2021 · Z97.5 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2022 edition of ICD-10-CM Z97.5 became effective on October 1, 2021. This is the American ICD-10-CM version of Z97.5 - other international versions of ICD-10 Z97.5 may differ. Type 1 Excludes.
What is the ICD 10 code for implantable contraceptive?
Jul 22, 2021 · #1 What is the ICD 10 dx code for a nexplanon implant status? A ahguzman Networker Messages 60 Location Florence, SC Best answers 0 Feb 13, 2019 #2 There isn't one specifically for subdermal contraceptive. Z97.8 presence of other specified device or Z30.46 if for surveillance- checking, reinsertion or removal. ahg CPC, CPMA, CGSC, COBGC KellyLR W
What is the CPT code for Breakthrough bleeding on the Nexplanon?
Oct 01, 2018 · J7307 Nexplanon ® ICD-10-PCS codes: OJHD3HZ Insertion of Contraceptive Device into Right Upper Arm OJHF3HZ. Insertion of Contraceptive Device into Left Upper Arm Providers may resubmit claims with dates of service on or after Oct. 1, 2018 to receive the appropriate reimbursement.
What happens if Nexplanon is not inserted correctly?
Oct 01, 2021 · 2022 ICD-10-CM Diagnosis Code Z30.46 2022 ICD-10-CM Diagnosis Code Z30.46 Encounter for surveillance of implantable subdermal contraceptive 2017 - New Code 2018 2019 2020 2021 2022 Billable/Specific Code POA Exempt Z30.46 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes.
What is the ICD 10 code for presence of NEXPLANON?
Presence of (intrauterine) contraceptive device Z97. 5 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2022 edition of ICD-10-CM Z97. 5 became effective on October 1, 2021.
What is the ICD 10 code for presence of subdermal contraceptive implant?
V45.52V45. 52 - Presence of subdermal contraceptive implant. ICD-10-CM.
How do you code a NEXPLANON?
The insertion and/or removal of the implant are reported using one of the following CPT (Current Procedural Terminology) codes:11981 Insertion, non-biodegradable drug delivery implant.11982 Removal, non-biodegradable drug delivery implant.11983 Removal with reinsertion, non-biodegradable drug delivery implant.
What is diagnosis code Z30 46?
Encounter for surveillance of implantable subdermal contraceptive46: Encounter for surveillance of implantable subdermal contraceptive.
Is NEXPLANON a subdermal implant?
Nexplanon is a long-acting hormonal contraceptive. A single implant is inserted subdermally and can be left in place for three years. Remove the implant no later than three years after the date of insertion. The user should be informed that she can request the removal of the implant at any time.
Is NEXPLANON an IUD?
is NEXPLANON an IUD? No, it's not an intrauterine device (IUD), because it's placed in your arm, not your uterus. But like an IUD, it's a long-acting birth control option because it lasts for 3 years.
What is the NDC number for nexplanon?
Contraceptive ImplantCPT CodeDescription of what you didHCPCS – J CodeBrand NameNDC NumberJ7307Nexplanon00052433001 00052027401bICD-10 CMDescription of why you did the insertionZ30.017Encounter for initial prescription of implantable subdermal implant3 more rows
What is procedure code 11983?
CPT Code 11983 Removal and re-insertion of single non-biodegradable implant.
What is diagnosis code Z30 49?
Encounter for surveillance of other contraceptives2022 ICD-10-CM Diagnosis Code Z30. 49: Encounter for surveillance of other contraceptives.
What is procedure code J7307?
J7307 - Etonogestrel (contraceptive) implant system, including implant and supplies.
What is subdermal implant?
Subdermal contraceptive implants involve the delivery of a steroid progestin from polymer capsules or rods placed under the skin. The hormone diffuses out slowly at a stable rate, providing contraceptive effectiveness for 1-5 years. The period of protection depends upon the specific progestin and the type of polymer.
Where is nexplanon inserted?
NEXPLANON is placed discreetly just under the skin of your non-dominant upper arm by a trained healthcare provider. During this minor surgical procedure, the area is numbed, and an applicator guides NEXPLANON into place.
What happens if you insert Nexplanon?
If NEXPLANON is inserted deeply (intramuscular or in the fascia), neural or vascular injury may occur.
When should Nexplanon be removed?
NEXPLANON should be removed in the event of a thrombosis. Due to the risk of thromboembolism associated with pregnancy and immediately following delivery, NEXPLANON should not be used prior to 21 days postpartum. Women with a history of thromboembolic disorders should be made aware of the possibility of a recurrence.
Can etonogestrel cause pregnancy?
Undetected failure to insert the implant may lead to an unintended pregnancy. Failure to remove the implant may result in continued effects of etonogestrel, such as compromised fertility, ectopic pregnancy, or persistence or occurrence of a drug-related adverse event.
Can a nexplanon be palpable?
NEXPLANON should be inserted subdermally so that it will be palpable after insertion, and this should be confirmed by palpation immediately after insertion. Failure to insert NEXPLANON properly may go unnoticed unless it is palpated immediately after insertion. Undetected failure to insert the implant may lead to an unintended pregnancy. Failure to remove the implant may result in continued effects of etonogestrel, such as compromised fertility, ectopic pregnancy, or persistence or occurrence of a drug-related adverse event.
What is CPT code for nexplanon?
11981 The insertion and/or removal of the implant are reported using one of the following CPT (Current Procedural Terminology) codes: 11981 Insertion, non-biodegradable drug delivery implant. 11982 Removal, non-biodegradable drug delivery implant. 11983 Removal with reinsertion, non-biodegradable drug delivery implant.
What is CPT code J7307?
J7307 – Etonogestrel (contraceptive) implant system, including implant and supplies.
What is DX code Z30 017?
Encounter for initial prescription of implantable subdermal contraceptive 2022 ICD-10-CM Diagnosis Code Z30. 017: Encounter for initial prescription of implantable subdermal contraceptive.
What is procedure code 11983?
CPT® 11983, Under Introduction or Removal Procedures on the Integumentary System. The Current Procedural Terminology (CPT®) code 11983 as maintained by American Medical Association, is a medical procedural code under the range – Introduction or Removal Procedures on the Integumentary System.
What is diagnosis code Z30 46?
46: Encounter for surveillance of implantable subdermal contraceptive.
What is CPT code J7300?
HCPCS code J7300 (intrauterine copper contraceptive [Paragard®] [10 year duration]) is reported for the IUD supply. The modifier 51 (multiple procedures) is added to CPT code 58300 to indicate the additional procedure (IUD insertion) performed at the same session as the primary procedure (delivery).
What is CPT code J7297?
2021 HCPCS Code J7297 : Levonorgestrel-releasing intrauterine contraceptive system (liletta), 52 mg.
What is the ICd 10 code for subcutaneous contraception?
Z30.46 is a billable diagnosis code used to specify a medical diagnosis of encounter for surveillance of implantable subdermal contraceptive. The code Z30.46 is valid during the fiscal year 2021 from October 01, 2020 through September 30, 2021 for the submission of HIPAA-covered transactions.#N#The ICD-10-CM code Z30.46 might also be used to specify conditions or terms like insertion of subcutaneous contraceptive done, subcutaneous contraceptive implant palpable, subcutaneous contraceptive implant present, surveillance of contraception done or surveillance of subcutaneous contraceptive implant done. The code is exempt from present on admission (POA) reporting for inpatient admissions to general acute care hospitals.#N#The code Z30.46 is applicable to female patients only. It is clinically and virtually impossible to use this code on a non-female patient.
What are the different types of birth control?
Types include birth control pills, patches, shots, vaginal rings, and emergency contraceptive pills. IUDs, devices which are implanted into the uterus. They can be kept in place for several years. Sterilization, which permanently prevents a woman from getting pregnant or a man from being able to get a woman pregnant.
What are the factors that determine birth control?
These include your health, frequency of sexual activity, number of sexual partners and desire to have children in the future. Your health care provider can help you select the best form of birth control for you.
Contraindications
- NEXPLANON should not be used in women who have known or suspected pregnancy; current or past history of thrombosis or thromboembolic disorders; liver tumors, benign or malignant, or active liver di...
Warnings and Precautions
- Complications of Insertion and Removal 1. NEXPLANON should be inserted subdermally so that it will be palpable after insertion, and this should be confirmed by palpation immediately after insertion. Failure to insert NEXPLANON properly may go unnoticed unless it is palpated immediately after insertion. Undetected failure to insert the implant may lead to an unintended pr…
Adverse Reactions
- Clinical Trial Experience 1. The most common adverse reaction causing discontinuation of use of the implant in clinical trials was change in menstrual bleeding patterns, specifically irregular menses (11.1%). The most common adverse reactions (≥10%) reported in clinical trials were headache (24.9%), vaginitis (14.5%), weight increase (13.7%), acne (13.5%), breast pain (12.8%…
Use in Specific Populations
- Pregnancy 1. Rule out pregnancy before inserting NEXPLANON. Lactation 1. Small amounts of contraceptive steroids and/or metabolites, including etonogestrel are present in human milk. No significant adverse effects have been observed in the production or quality of breast milk, or on the physical and psychomotor development of breastfed infants. 2. Hormonal contraceptives, inc…
Popular Posts:
- 1. icd 10 code for probable pancreatic cancer
- 2. icd-10 code for elevated protein
- 3. icd 10 code for struck by ball
- 4. icd 10 code for articulation disorder
- 5. 2015 icd 10 code for post op amputation foot
- 6. icd 10 code for hypotonia
- 7. what is the icd 10 code for vt\
- 8. icd 10 code for v02.61
- 9. icd-10 code for left middle finger injury
- 10. icd 10 code for left radius fracture