What is the ICD 10 code for fecal abnormalities?
Other fecal abnormalities. R19.5 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2018/2019 edition of ICD-10-CM R19.5 became effective on October 1, 2018.
Is fecal immunochemical testing cost‐effective for colorectal cancer screening?
Annual fecal immunochemical testing (FIT) is cost‐effective for colorectal cancer (CRC) screening. However, FIT positivity rates and positive predictive value (PPV) can vary substantially, with false‐positive (FP) results adding to colonoscopy burden without improving cancer detection.
How should I prepare for a fecal immunochemical test (fit)?
For the fecal immunochemical test (FIT), which is the preferred test, no preparation is necessary. For the guaiac-based FOBT (gFOBT, FOBT), you will be instructed to avoid certain medications and follow certain dietary restrictions for several days before collecting the stool samples.
What is the ICD 10 code for abnormal stool color?
Diagnosis Index entries containing back-references to R19.5: Abnormal, abnormality, abnormalities - see also Anomaly stool (color) (contents) (mucus) R19.5 guaiac positive R19.5 Blood in feces K92.1 ICD-10-CM Diagnosis Code K92.1 Bulky stools R19.5 Fat in stool R19.5 Mucus in stool R19.5 Occult blood in feces R19.5 (stools) Pus in stool R19.5
What is the ICD-10 code for positive fecal occult blood test?
ICD-10 Code for Other fecal abnormalities- R19. 5- Codify by AAPC.
What is the ICD-10 code for heme positive stool?
2022 ICD-10-CM Diagnosis Code K92. 1: Melena.
What is the ICD-10 code for large stool burden?
K56. 41 - Fecal impaction | ICD-10-CM.
What is the ICD-10 code for elevated calprotectin?
APPLICABLE CODESICD-10 Diagnosis CodeDescriptionK50.014Crohn's disease of small intestine with abscessK50.018Crohn's disease of small intestine with other complicationK50.019Crohn's disease of small intestine with unspecified complicationsK50.10Crohn's disease of large intestine without complications71 more rows
What does code Z12 11 mean?
A screening colonoscopy should be reported with the following International Classification of Diseases, 10th edition (ICD-10) codes: Z12. 11: Encounter for screening for malignant neoplasm of the colon.
What is the diagnosis code for stool in colon?
K56. 41 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2022 edition of ICD-10-CM K56. 41 became effective on October 1, 2021.
What is the diagnosis for ICD-10 code r50 9?
9: Fever, unspecified.
What is the ICD-10-CM code for loose stools?
ICD-10-CM Code for Diarrhea, unspecified R19. 7.
What does large stool burden mean?
If you often have trouble making bowel movements and have to take laxatives (drugs that help you go) on a regular basis, you could one day have a serious bowel problem called fecal impaction. A fecal impaction is a large, hard mass of stool that gets stuck so badly in your colon or rectum that you can't push it out.
What does elevated calprotectin mean?
High levels of calprotectin in stool may signal IBD, colorectal cancer, or infection. Moderate or low levels mean there's little to no inflammation present in the intestines. This may indicate that your symptoms are caused by a viral infection or IBS.
What is calprotectin in stool?
A calprotectin stool test detects levels of calprotectin, which is a protein that indicates bowel inflammation, in a person's stool. Although doctors typically perform these tests to help diagnose inflammatory bowel disease (IBD), they may also use them to track the development of an existing IBD diagnosis.
What causes raised calprotectin?
Having a raised calprotectin level generally means you have active inflammation in your body. This is generally associated with inflammatory bowel diseases (IBD) such as Crohn's disease or ulcerative colitis. The higher the level of faecal calprotectin the more inflammation present in your intestines.
What is the CPT code for colonoscopy?
Effective January 1, 2018, anesthesia services furnished in conjunction with and in support of a screening colonoscopy are reported with CPT code 00812 and coinsurance and deductible are waived. When a screening colonoscopy becomes a diagnostic colonoscopy, anesthesia services are reported with CPT code 00811 and with the PT modifier; only the deductible is waived.
What is the sensitivity of a blood based screening test?
proven test performance characteristics for a blood-based screening test with both sensitivity greater than or equal to 74% and specificity greater than or equal to 90% in the detection of colorectal cancer compared to the recognized standard (accepted as colonoscopy at this time), as minimal threshold levels, based on the pivotal studies included in the FDA.
How often is a biomarker test required for Medicare?
Effective for dates of service on or after January 19, 2021, a blood-based biomarker test is covered as an appropriate colorectal cancer screening test once every 3 years for Medicare beneficiaries when performed in a Clinical Laboratory Improvement Act (CLIA)-certified laboratory, when ordered by a treating physician and when all of the following requirements are met:
When did CPT 00810 become effective?
Effective January 1, 2015 through December 31, 2017, anesthesia professionals who furnish a separately payable anesthesia service (CPT code 00810) in conjunction with a screening colonoscopy shall include the following on the claim for the services that qualify for the waiver of coinsurance and deductible:
What happens if you submit a claim without a diagnosis code?
A claim submitted without a valid ICD-10-CM diagnosis code will be returned to the provider as an incomplete claim under Section 1833 (e) of the Social Security Act.
Is a PT deductible waived for a colonoscopy?
Effective January 1, 2018, coinsurance and deduct ible are waived for moderate sedation services (reported with G0500 or 99153) when furnished in conjunction with and in support of a screening colonoscopy service and when reported with modifier 33. When a screening colonoscopy becomes a diagnostic colonoscopy, moderate sedation services (G0500 or 99153) are reported with only the PT modifier; only the deductible is waived.
What foods can cause a positive blood test?
The test relies on a chemical reaction to produce the color change that gives a positive test. Foods such as broccoli, turnips, cauliflower and apples, and drugs such as colchicine may make the test appear positive even in the absence of blood (a false-positive result).
How to test for guaiac?
Stool should be collected into clean containers and should not be contaminated with urine or water. Using an applicator stick, a sample is collected from the surface of the stool and placed onto the specially treated pad on the test card and allowed to dry. When using cards with multiple test areas, the samples for each test area are collected on different days. For example, a 3-section card requires 3 stools, each collected on a different day. Collecting and testing multiple stool samples increases the chance of detecting cancer if it is present.
What is the guaiac test?
The guaiac-based tests (gFOBT, FOBT) measure the heme (non-protein) part of hemoglobin from blood in the stool. Since the heme part of hemoglobin is common ...
How to collect stool samples for FIT?
For FIT, a common approach is to use a brush or other device to collect a sample from the surface of a stool, which is then inserted into a sample tube containing a solution and sent for testing. Generally, a single sample is required.
Can occult blood be detected in stool?
This small amount of blood may be the first and sometimes the only sign of polyps or early colon cancer, making the stool-based tests valuable screening tools. There are two principal methods for detecting occult blood in the stool. They are designed to detect hemoglobin, a molecule that is present in red blood cells.
Do you need a stool sample?
Stool samples are required. The sampling procedure depends on the choice of test, and the samples may be collected in the privacy of your home. (See the section "What is being tested?" below for more details.)
How are colonoscopy results determined?
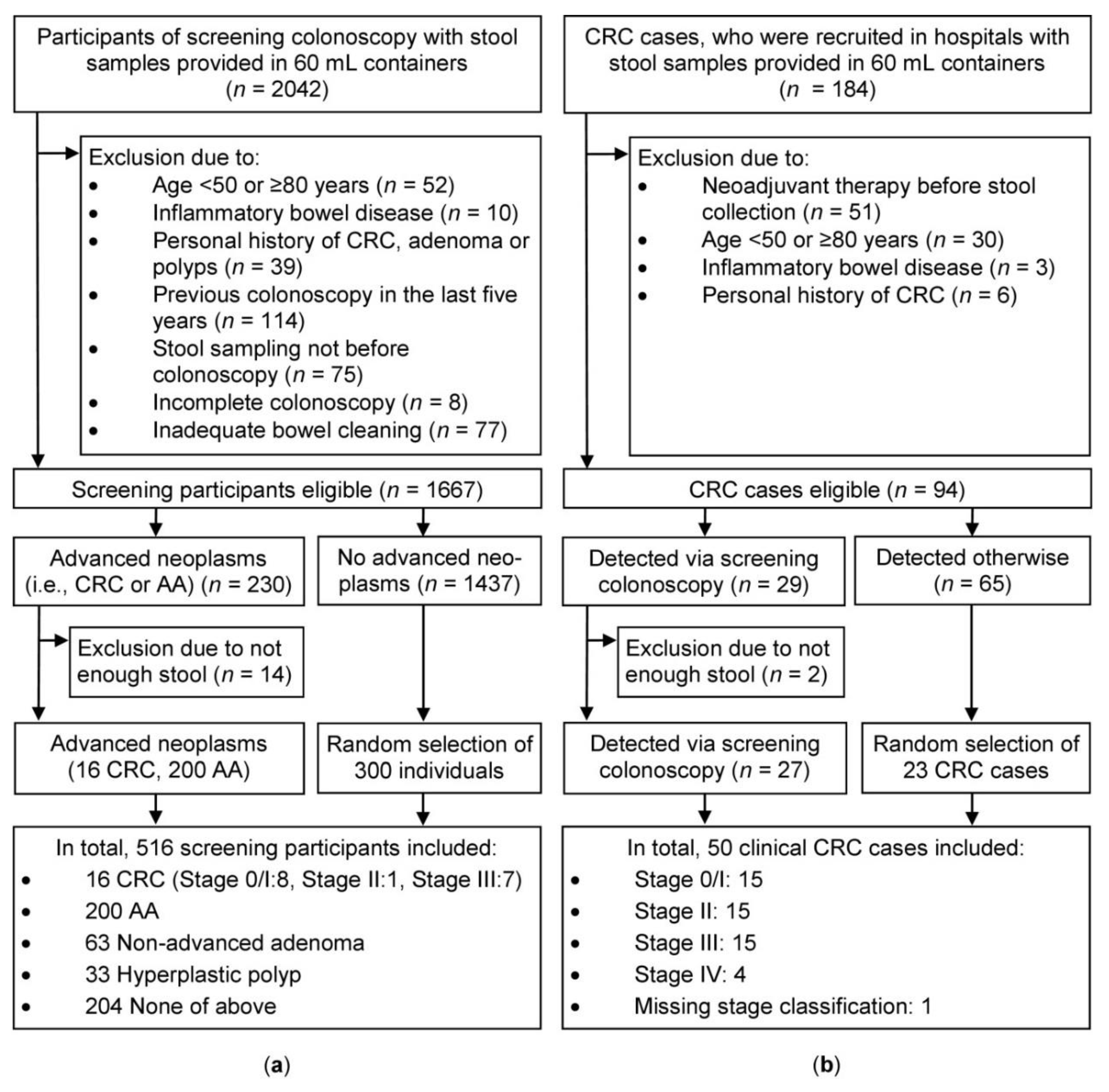
Colonoscopy results were determined through chart abstraction of the pathology or procedure report, when available, or through clinician notes . All charts were accessed in collaboration with OCHIN, a nonprofit health center network with an organization‐wide EHR that allows researchers to access clinical and utilization data across all OCHIN clinic sites. A trained abstractor collected data for the fields listed in Table S1.
What is the PPV for advanced neoplasia?
PPV for advanced neoplasia (including CRC or AA) also varied substantially (16% for the clinic that used Hemosure to 31% for those using OC‐Micro). These values are similar to those previously reported for advanced neoplasia26and are between those reported for CRC (2%‐17%)15, 26and advanced adenoma (35%‐51%).37, 38Moreover, in a sensitivity analysis that moved nonadvanced adenomas into the “positive” outcome, PPVs rose to 39‐53%. The substantial number of nonadvanced adenomas detected in clinics that used the InSure FIT led to a PPV that was significantly higher than with Hemosure or OC‐Micro. However, in multivariable analyses of false positivity, differences by FIT kit were not statistically significant. The large differences in PPV across definitions of a “positive” colonoscopic finding, both in our study and in previous literature, underscore the need to interpret screening test value in the context of follow‐up diagnostic and treatment measures appropriate to each specific outcome (CRC, AA, and nonadvanced adenoma).
Is smoking associated with false positivity?
Although smoking has been consistently associated with false positivity ( ORs from 1.3 to 1.7),21, 23, 24we saw no association. Smoking history was determined from social history fields in the EHR, and a substantial proportion (10%) were missing data on smoking status. The potential for misclassification and the effect of missing values may have affected our ability to detect an association.
Is bpathology report included in PPV?
bPathology report was unavailable in the patient's health record; therefore, the presence of polyp(s) was determined through provider notes. This category was not included in PPV calculations.
Does NSAID cause false positive?
Use of NSAIDs was not significantly associated with false positivity, which may be due to a lower CRC risk among NSAID users.45Previous studies have reported significant associations between use of antiplatelet medication (OR for false positivity ≈2.5)23or proton‐pump inhibitors (OR = 1.8).42We found no association between anticoagulant use and false positivity. Previous studies showed no negative impact of Warfarin on FIT test performance,46, 47while low‐dose aspirin was suggested to improve sensitivity.48In considering the body of evidence for anticoagulant use on FIT performance, the US Multi‐Society Task Force on Colorectal Cancer found no rationale for altering anticoagulant medication before FIT screening to improve PPV.2
Why is fecal test important?
The quality of fecal test results is important. However, given the lack of test performance data for the most commonly used test among these providers ( InSure), the need for better population-based test performance information and communication of that information to providers is apparent.
How are colonoscopy results determined?
Colonoscopy results were determined through chart abstraction of the pathology or procedure report, when available, or through clinician notes. All charts were accessed in collaboration with OCHIN, a nonprofit health center network with an organization-wide EHR that allows researchers to access clinical and utilization data across all OCHIN clinic sites. A trained abstractor collected data for the fields listed in Table S1 .
What is the PPV for advanced neoplasia?
PPV for advanced neoplasia (including CRC or AA) also varied substantially (16% for the clinic that used Hemosure to 31% for those using OC-Micro). These values are similar to those previously reported for advanced neoplasia 26 and are between those reported for CRC (2%-17%) 15, 26 and advanced adenoma (35%-51%). 37, 38 Moreover, in a sensitivity analysis that moved nonadvanced adenomas into the “positive” outcome, PPVs rose to 39-53%. The substantial number of nonadvanced adenomas detected in clinics that used the InSure FIT led to a PPV that was significantly higher than with Hemosure or OC-Micro. However, in multivariable analyses of false positivity, differences by FIT kit were not statistically significant. The large differences in PPV across definitions of a “positive” colonoscopic finding, both in our study and in previous literature, underscore the need to interpret screening test value in the context of follow-up diagnostic and treatment measures appropriate to each specific outcome (CRC, AA, and nonadvanced adenoma).
Is smoking associated with false positivity?
Although smoking has been consistently associated with false positivity ( ORs from 1.3 to 1.7), 21, 23, 24 we saw no association. Smoking history was determined from social history fields in the EHR, and a substantial proportion (10%) were missing data on smoking status. The potential for misclassification and the effect of missing values may have affected our ability to detect an association.
Does NSAID cause false positive?
Use of NSAIDs was not significantly associated with false positivity, which may be due to a lower CRC risk among NSAID users. 45 Previous studies have reported significant associations between use of antiplatelet medication (OR for false positivity ≈2.5) 23 or proton-pump inhibitors (OR = 1.8). 42 We found no association between anticoagulant use and false positivity. Previous studies showed no negative impact of Warfarin on FIT test performance, 46, 47 while low-dose aspirin was suggested to improve sensitivity. 48 In considering the body of evidence for anticoagulant use on FIT performance, the US Multi-Society Task Force on Colorectal Cancer found no rationale for altering anticoagulant medication before FIT screening to improve PPV. 2
Popular Posts:
- 1. icd 10 dx code for bilateral hip replacement
- 2. icd 10 code for hypertension with cad
- 3. icd 10 code for non intractable vomiting
- 4. icd 10 code for sickle cell trait in pregnancy
- 5. icd 10 code for scoliosis with comp
- 6. icd-10-cm code for gastroenteritis
- 7. icd 10 code for iodine unspecified
- 8. icd 10 code for sprain of left shoulder joint
- 9. icd 10 code for statin intolerance
- 10. icd 10 code for bilateral patellofemoral arthritis