What is the CPT code for tricuspid valve replacement?
02RJ3JZ is a billable procedure code used to specify the performance of replacement of tricuspid valve with synthetic substitute, percutaneous approach. The code is valid for the year 2022 for the submission of HIPAA-covered transactions.
What are the I07 and i08 tricuspid valve disorders?
tricuspid valve disorders of unspecified cause ( I07.-) tricuspid valve disorders specified as rheumatic ( I07.-) tricuspid valve disorders with aortic and/or mitral valve involvement ( I08.-)
What is the ICD 10 code for POA exempt?
2016 2017 2018 2019 Billable/Specific Code POA Exempt. Z95.2 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2018/2019 edition of ICD-10-CM Z95.2 became effective on October 1, 2018.
What is the ICD 10 code for implantable heart surgery?
Diagnosis Index entries containing back-references to Z95.2: Presence (of) artificial heart (fully implantable) (mechanical) Z95.812 ICD-10-CM Diagnosis Code Z95.812 Replacement by artificial or mechanical device or prosthesis of heart Z95.812 ICD-10-CM Diagnosis Code Z95.812
What is CTAMVI in surgery?
What is PMVR in medical terms?
What is tricuspid valve repair?
What is secondary MR?
How common is mitral valve regurgitation?
What is an active inflammation of the heart?
See more
About this website
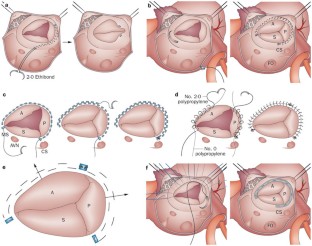
repair of tricuspid valve | Medical Billing and Coding Forum - AAPC
I need help coding a valve repair. I seem to have a problem understanding the annuloplasty with ring procedure. The procedure, as listed in the operative report is 1) tricuspid valve repair (Alfieri); 2) tricuspid valve annuloplasty with 33mm duran ring. I looked to repair tricuspud valve...
Mitral Valve Repair | Medical Billing and Coding Forum - AAPC
I had asked STS regarding the different approach/and bypass placement for the MVR. This was their response: Currently there is no code differential for the type of exposure associated with the the mitral valve repair, so you would just identify the code for the repair, and report that one.
2019 MITRACLIP CODING AND PAYMENT GUIDE
page 1 page 2 page 3 page 4 references and brief summary 3 physician claim checklist physician coding policy update hospital inpatient coding hospital inpatient payment
Tricuspid Clip in Tricuspid Regurgitation - American College of Cardiology
As part of American Heart Month, and in recognition of National Heart Valve Disease Awareness Day on Feb. 22, the ACC Interventional Section has written a series of articles focused on valvular heart disease, including the role of imaging modalities, emerging trends in transcatheter mitral valve replacement and tricuspid valve repair, and perspectives on novel applications of transcatheter ...
Percutaneous Tricuspid Valve Repair - StatPearls - NCBI Bookshelf
Tricuspid valve regurgitation and stenosis are rare valvular heart diseases. Both can be treated medically but often require more invasive intervention such as surgical repair of the valve. Percutaneous intervention, or transcatheter valve repair, has become a more popular method than surgery for nonsurgical candidates in recent years. Over the last several years, percutaneous intervention on ...
What is CTAMVI in surgery?
Ando and colleagues (2017) stated that combined transcatheter aortic and mitral valve intervention (CTAMVI), a combination of either transcatheter aortic valve replacement (TAVR) or transcatheter aortic valve-in-valve (TAViV) and TMVR, transcatheter mitral ViV/ViR (TMViV/ViR), or PMVR is an attractive alternative in high-surgical risk patients with combined aortic and mitral valve disease. However, its procedural details and clinical outcomes have not been well described. These investigators summarized the published data of CTAMVI. They performed a systematic review of all the published articles from PubMed and Embase. A total of 37 studies with 60 patients were included. The indication for CTAMVI was high or inoperable surgical risk and symptomatic severe aortic stenosis (92 %) or severe aortic regurgitation (8 %) combined with moderate-to-severe/severe mitral stenosis (30 %) or moderate/severe MR (65 %) or both (5 %). In majority of the cases, aortic valve intervention was performed prior to the mitral valve. Mortality rate were 25 % for TAVR + TMVR (range of 42 days to 10 months), 17 % for TAVR + TMViV/ViR (range of 13 days to 6 months), 0 % for TAViV + TMViV/ViR (range of 6 to 365 days), and 15 % for TAVR/ViV + PMVR (range of 17 days to 419 days). Significant (more than moderate) para-valvular regurgitation post-procedure was rare. The authors concluded that CTAMVI appeared to confer reasonable clinical outcome. Moreover, they stated that further large clinical trials are needed to clarify the optimal strategy, procedural details and clinical outcomes in the future.
What is PMVR in medical terms?
Aetna considers percutaneous mitral valve repair (PMVR) by means of the MitraClip Clip Delivery System medically necessary for persons with grade 3+ to 4+ symptomatic degenerative mitral regurgitation and at high-risk for traditional open-heart mitral valve surgery.
What is tricuspid valve repair?
Tricuspid valve repair or replacement via a transcatheter approach, devices for transcatheter tricuspid valve repair (T TVR) and replacement are in early stages of development for the treatment of TR. There are early studies evaluating use of 2 TTVR devices, the TriClip Delivery System, essentially the same clip delivery used for the mitral valve and the Cardioband Valve System delivery via transfemoral approach (TRI-REPAIR Study). Individual selection criteria for percutaneous tricuspid valve replacement are based on limited data. Currently there are no FDA-approved devices to be delivered in the tricuspid position.
What is secondary MR?
Lavall and co-workers (2018) noted that secondary MR results from LV dilatation and dysfunction. Quantification of secondary MR is challenging because of the underlying myocardial disease. Clinical and echocardiographic evaluation requires a multi-parametric approach. Severe secondary MR occurs in up to 25 % of patients with HF with reduced ejection fraction, which is associated with a mortality rate of 40 % to 50 % in 3 years. Percutaneous edge-to-edge mitral valve repair (MitraClip) has emerged as an alternative to surgical valve repair to improve symptoms, functional capacity, HF hospitalizations, and cardiac hemodynamic. Further new transcatheter strategies addressing MR are evolving. The Carillion, Cardioband, and Mitralign devices were designed to reduce the annulus dilatation, which is a frequent and important determinant of secondary MR. Several transcatheter mitral valve replacement systems (Tendyne, CardiAQ-Edwards, Neovasc, Tiara, Intrepid, Caisson, HighLife, MValve System, and NCSI NaviGate Mitral) are emerging because valve replacement might be more durable compared with valve repair. In small studies, these interventional therapies demonstrated feasibility and efficiency to reduce MR and to improve HF symptoms. However, neither transcatheter nor surgical mitral valve repair or replacement has been proven to impact on the prognosis of HF patients with severe MR, which remains high with a mortality rate of 14 % to 20 % at 1 year. To-date, the primary indication for treatment of secondary severe MR is the amelioration of symptoms, reinforcing the value of a Heart Team discussion. The authors concluded that randomized studies examining the treatment effect and long-term outcome for any transcatheter or surgical mitral valve intervention compared with optimized medical treatment are needed and underway.
How common is mitral valve regurgitation?
Mitral valve regurgitation, for which surgical mitral valve repair is the treatment of choice, is the second most common clinically relevant valvular heart disease in adults and has an incidence of approximately 2 % to 3 % per year (Seeburger et al, 2011).
What is an active inflammation of the heart?
Persons with active inflammation of the heart (endocarditis) Persons with blood clots present at the intended site of implant or blood clots in vessels through which access to the defect is gained. Persons with mitral regurgitation who can be treated with open-heart surgery. Person with rheumatic mitral valve disease.
What is CTAMVI in surgery?
Ando and colleagues (2017) stated that combined transcatheter aortic and mitral valve intervention (CTAMVI), a combination of either transcatheter aortic valve replacement (TAVR) or transcatheter aortic valve-in-valve (TAViV) and TMVR, transcatheter mitral ViV/ViR (TMViV/ViR), or PMVR is an attractive alternative in high-surgical risk patients with combined aortic and mitral valve disease. However, its procedural details and clinical outcomes have not been well described. These investigators summarized the published data of CTAMVI. They performed a systematic review of all the published articles from PubMed and Embase. A total of 37 studies with 60 patients were included. The indication for CTAMVI was high or inoperable surgical risk and symptomatic severe aortic stenosis (92 %) or severe aortic regurgitation (8 %) combined with moderate-to-severe/severe mitral stenosis (30 %) or moderate/severe MR (65 %) or both (5 %). In majority of the cases, aortic valve intervention was performed prior to the mitral valve. Mortality rate were 25 % for TAVR + TMVR (range of 42 days to 10 months), 17 % for TAVR + TMViV/ViR (range of 13 days to 6 months), 0 % for TAViV + TMViV/ViR (range of 6 to 365 days), and 15 % for TAVR/ViV + PMVR (range of 17 days to 419 days). Significant (more than moderate) para-valvular regurgitation post-procedure was rare. The authors concluded that CTAMVI appeared to confer reasonable clinical outcome. Moreover, they stated that further large clinical trials are needed to clarify the optimal strategy, procedural details and clinical outcomes in the future.
What is PMVR in medical terms?
Aetna considers percutaneous mitral valve repair (PMVR) by means of the MitraClip Clip Delivery System medically necessary for persons with grade 3+ to 4+ symptomatic degenerative mitral regurgitation and at high-risk for traditional open-heart mitral valve surgery.
What is tricuspid valve repair?
Tricuspid valve repair or replacement via a transcatheter approach, devices for transcatheter tricuspid valve repair (T TVR) and replacement are in early stages of development for the treatment of TR. There are early studies evaluating use of 2 TTVR devices, the TriClip Delivery System, essentially the same clip delivery used for the mitral valve and the Cardioband Valve System delivery via transfemoral approach (TRI-REPAIR Study). Individual selection criteria for percutaneous tricuspid valve replacement are based on limited data. Currently there are no FDA-approved devices to be delivered in the tricuspid position.
What is secondary MR?
Lavall and co-workers (2018) noted that secondary MR results from LV dilatation and dysfunction. Quantification of secondary MR is challenging because of the underlying myocardial disease. Clinical and echocardiographic evaluation requires a multi-parametric approach. Severe secondary MR occurs in up to 25 % of patients with HF with reduced ejection fraction, which is associated with a mortality rate of 40 % to 50 % in 3 years. Percutaneous edge-to-edge mitral valve repair (MitraClip) has emerged as an alternative to surgical valve repair to improve symptoms, functional capacity, HF hospitalizations, and cardiac hemodynamic. Further new transcatheter strategies addressing MR are evolving. The Carillion, Cardioband, and Mitralign devices were designed to reduce the annulus dilatation, which is a frequent and important determinant of secondary MR. Several transcatheter mitral valve replacement systems (Tendyne, CardiAQ-Edwards, Neovasc, Tiara, Intrepid, Caisson, HighLife, MValve System, and NCSI NaviGate Mitral) are emerging because valve replacement might be more durable compared with valve repair. In small studies, these interventional therapies demonstrated feasibility and efficiency to reduce MR and to improve HF symptoms. However, neither transcatheter nor surgical mitral valve repair or replacement has been proven to impact on the prognosis of HF patients with severe MR, which remains high with a mortality rate of 14 % to 20 % at 1 year. To-date, the primary indication for treatment of secondary severe MR is the amelioration of symptoms, reinforcing the value of a Heart Team discussion. The authors concluded that randomized studies examining the treatment effect and long-term outcome for any transcatheter or surgical mitral valve intervention compared with optimized medical treatment are needed and underway.
How common is mitral valve regurgitation?
Mitral valve regurgitation, for which surgical mitral valve repair is the treatment of choice, is the second most common clinically relevant valvular heart disease in adults and has an incidence of approximately 2 % to 3 % per year (Seeburger et al, 2011).
What is an active inflammation of the heart?
Persons with active inflammation of the heart (endocarditis) Persons with blood clots present at the intended site of implant or blood clots in vessels through which access to the defect is gained. Persons with mitral regurgitation who can be treated with open-heart surgery. Person with rheumatic mitral valve disease.
Popular Posts:
- 1. icd 10 code for m54.16
- 2. icd 10 code for bilateral back pain
- 3. icd 10 code for acquired soft tissue deformity of ear
- 4. icd 10 code for spontaneous rupture of posterior tibial tendon
- 5. icd 10 code for basic metabolic panel
- 6. icd 10 code for right adrilles tendon pain
- 7. icd 10 code for displaced ulna fracture
- 8. icd 10 code for stage 3 pressure ulcer coccyx
- 9. icd 10 code for child sports physical
- 10. icd 10 code for lateral stemi