62350, implantation, revision, repositioning of tunneled intrathecal or epidural catheter, no laminectomy 62362, includes preparation of pump, with or without programming Removal 62355, removal of a previously implanted intrathecal or epidural catheter 62365, removal of subcutaneous reservoir or pump
Full Answer
What is the ICD 10 code for intrathecal infusion pump?
This is the American ICD-10-CM version of T85.695 - other international versions of ICD-10 T85.695 may differ. Applicable To. Other mechanical complication of intrathecal infusion pump. The following code (s) above T85.695 contain annotation back-references. Annotation Back-References.
What is the C code for removal of previously implanted intrathecal catheter?
CPT 62355: Removal of previously implanted intrathecal or epidural catheter. (10 days Global) Note: Medicare provides C-codes for hospital use in billing Medicare for medical devices in the outpatient setting. Although other payers may also accept C-codes, regular HCPCS II device codes are generally used for billing non-Medicare payers.
What is an intrathecal pump system?
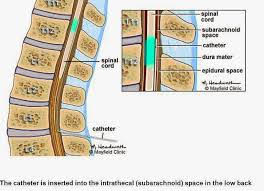
The intrathecal pump system consists of a pump/reservoir implanted between the muscle and skin of your abdomen and a catheter that carries pain medication from the pump to the spinal cord and nerves. The pump is programmed to slowly release medication over a period of time.
What is the ICD 9 code for medical coding?
ICD-9-CM V53.09 is a billable medical code that can be used to indicate a diagnosis on a reimbursement claim, however, V53.09 should only be used for claims with a date of service on or before September 30, 2015. For claims with a date of service on or after October 1, 2015, use an equivalent ICD-10-CM code (or codes).
What is the ICD 10 code for presence of intrathecal pump?
Encounter for adjustment and management of infusion pump Z45. 1 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2022 edition of ICD-10-CM Z45. 1 became effective on October 1, 2021.
What is the CPT code for intrathecal pump trial?
*CPT® codes 62322, 62323, 62326 and 62327 should be reported when performing trial infusions prior to permanent pump implantation.
What is the ICD 10 code for long term use of morphine?
ICD-10 code Z79. 891 for Long term (current) use of opiate analgesic is a medical classification as listed by WHO under the range - Factors influencing health status and contact with health services .
What is the CPT code for an implantation or replacement of device for intrathecal drug infusion non programmable pump?
62361 (Implantation or replacement of device for intrathecal or epidural drug infusion; nonprogrammable pump).
How much does an intrathecal pain pump weigh?
It weighs about 6 ounces. It is implanted just under the skin of the abdomen (Picture 1). The battery-powered device stores the medicine. The catheter is a flexible tube that connects to the pump.
What is the ICD 10 code for intrathecal pain pump?
Pain due to nervous system prosthetic devices, implants and grafts, subsequent encounter. T85. 840D is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2022 edition of ICD-10-CM T85.
What is the CPT code 62323?
62323. Injection(s), of diagnostic or therapeutic substance(s) (eg, anesthetic, antispasmodic, opioid, steroid, other solution), not including. neurolytic substances, including needle or catheter placement, interlaminar epidural or subarachnoid, lumbar or sacral (caudal); with.
What is diagnosis code Z51 81?
ICD-10 code Z51. 81 for Encounter for therapeutic drug level monitoring is a medical classification as listed by WHO under the range - Factors influencing health status and contact with health services .
What is the ICD-10 code for F11 90?
Table 4ICD-9-CM and ICD-10-CM diagnosis codes defining opioid use disorder (OUD)Diagnosis codeDescriptionICD-9-CM diagnosis codesF11.90Opioid use, unspecified, uncomplicatedF11.920Opioid use, unspecified with intoxication, uncomplicatedF11.921Opioid use, unspecified with intoxication delirium138 more rows
What is the ICD-10 code for V58 69?
V58. 69 - Long-term (current) use of other medications. ICD-10-CM.
What is the difference between 62369 and 62370?
Both codes were added to the coding family to describe electronic analysis with reprogramming and refill. Code 62369 is reported when physician skill is not required to reprogram and refill. Code 62370 is reported when reprogramming and refill require physician's skill.
What is procedure code 62370?
CPT® Code 62370 in section: Electronic analysis of programmable, implanted pump for intrathecal or epidural drug infusion (includes evaluation of reservoir status, alarm status, drug prescription status)
What is procedure code 62362?
CPT® Code 62362 in section: Implantation or replacement of device for intrathecal or epidural drug infusion.
Is Dilaudid used in pain pumps?
Medications used in your pump include: Opioids – Morphine and Hydromorphone (Dilaudid) are often used. Local anesthetics (i.e. Bupivacaine) – This medicine blocks pain signals in the spinal cord.
What is a pain pump called?
A pain pump, also known as an intrathecal pump implant, offer patients medication directly at the source of pain.
What is an intrathecal pain pump?
An intrathecal pump or a "pain pump" is a device that delivers small quantities of pain medication such as morphine or baclofen, directly to the spinal fluid. When delivered in small doses, pain medications may minimize the side effects often experienced with larger oral doses of the same medications.
General Information
CPT codes, descriptions and other data only are copyright 2021 American Medical Association. All Rights Reserved. Applicable FARS/HHSARS apply.
Article Guidance
An implanted infusion pump for chronic pain is covered by Medicare when used to 1) administer opioid drugs, singly or in combination with other opioid or non-opioid drugs, 2) intrathecal or epidural route; 3) for treatment of severe chronic intractable pain of malignant or nonmalignant origin in patients who have a life expectancy of at least three (3) months, and 4) the pain has been proven to be unresponsive to less invasive medical therapy. In order to be considered medically reasonable and necessary, all of the following criteria must be met and clearly documented in the beneficiary’s medical record:.
Bill Type Codes
Contractors may specify Bill Types to help providers identify those Bill Types typically used to report this service. Absence of a Bill Type does not guarantee that the article does not apply to that Bill Type.
Revenue Codes
Contractors may specify Revenue Codes to help providers identify those Revenue Codes typically used to report this service. In most instances Revenue Codes are purely advisory. Unless specified in the article, services reported under other Revenue Codes are equally subject to this coverage determination.
Indications and Usage
Lioresal ® Intrathecal (baclofen injection) is a muscle relaxant and antispastic that is indicated for use in the management of severe spasticity of cerebral or spinal origin.
Select Warnings and Precautions
It is mandatory that all patients, caregivers, and treating physicians receive adequate information regarding the risks of the mode of treatment.
Adverse Reactions
The most frequent drug adverse events vary by indication but include: hypotonia (34.7%), somnolence (20.9%), headache (10.7%), convulsion (10.0%), dizziness (8.0%), urinary retention (8.0%), nausea (7.3%), and paresthesia (6.7%). Dosing and programming errors may result in clinically significant overdose or withdrawal.
Use in Specific Populations
There are no adequate and well controlled studies in pregnant women. Lioresal ® Intrathecal should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
What is the ICd 10 code for mechanical complication?
Other mechanical complication of other nervous system device, implant or graft 1 T85.695 should not be used for reimbursement purposes as there are multiple codes below it that contain a greater level of detail. 2 Short description: Mech compl of other nervous system device, implant or graft 3 The 2021 edition of ICD-10-CM T85.695 became effective on October 1, 2020. 4 This is the American ICD-10-CM version of T85.695 - other international versions of ICD-10 T85.695 may differ.
What is the secondary code for Chapter 20?
Use secondary code (s) from Chapter 20, External causes of morbidity, to indicate cause of injury. Codes within the T section that include the external cause do not require an additional external cause code. Type 1 Excludes.
Popular Posts:
- 1. icd 10 code for colostomy complication
- 2. icd 10 code for right upper quadrant
- 3. icd 10 code for r foot osteomyelitis
- 4. icd 10 code for blepharitis left upper eyelid
- 5. icd 10 code for crushing injury to righttoe
- 6. icd 9 code for vre
- 7. icd 10 code for hypovolemic _____ septic shock
- 8. icd 10 code for fall on ground
- 9. icd 10 code for bmi 47
- 10. icd 10 code for polyosteoarthritis multiple sites