What is the ICD 10 code for periorbit rhytides?
Oct 01, 2021 · Rhytides and wrinkles, glabellar Skin disorder, degenerative Vascular disease of skin Vascular disease of the skin Venous lake Volume loss face Volume loss of face Wrinkled skin Wrinkles Wrinkles, perioral (around the mouth) ICD-10-CM L98.8 is grouped within Diagnostic Related Group (s) (MS-DRG v39.0): 606 Minor skin disorders with mcc
What is the ICD 10 code for rhytidosis facialis?
Oct 01, 2021 · Periorbit rhytides (eye condition) ICD-10-CM L90.8 is grouped within Diagnostic Related Group(s) (MS-DRG v 39.0): 606 Minor skin disorders with mcc; 607 Minor skin disorders without mcc; Convert L90.8 to ICD-9-CM. Code History. 2016 (effective 10/1/2015): New code (first year of non-draft ICD-10-CM) 2017 (effective 10/1/2016): No change
What is the ICD-10-CM diagnosis code for macule?
R23.4 is a billable diagnosis code used to specify a medical diagnosis of changes in skin texture. The code R23.4 is valid during the fiscal year 2022 from October 01, 2021 through September 30, 2022 for the submission of HIPAA-covered transactions. The ICD-10-CM code R23.4 might also be used to specify conditions or terms like abnormal desquamation, abnormal keratinization, …
What is the ICD 10 code for disrd of the skin?
Rhytidosis Facialis ICD-10-CM Alphabetical Index The ICD-10-CM Alphabetical Index is designed to allow medical coders to look up various medical terms and connect them with the appropriate ICD codes. There are 0 terms under the parent term 'Rhytidosis Facialis' in the ICD-10-CM Alphabetical Index . Rhytidosis Facialis See Code: L98.8
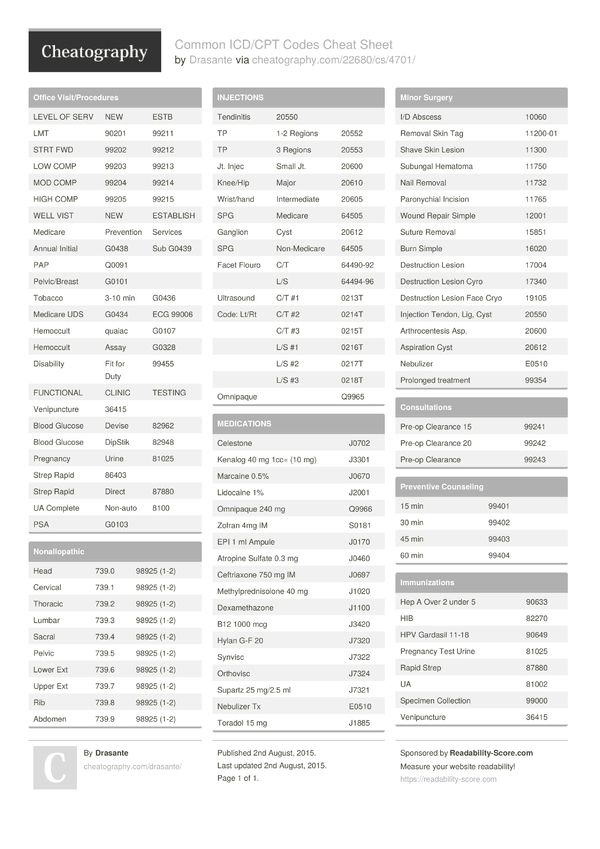
What is the diagnosis code for Panniculectomy?
One code, CPT 15830 for panniculectomy, can be billed to insurance when appropriate; the other code, CPT 15847 for abdominoplasty, describes a cosmetic procedure and therefore should not be billed to insurance.
What is L98 8?
ICD-10 code L98. 8 for Other specified disorders of the skin and subcutaneous tissue is a medical classification as listed by WHO under the range - Diseases of the skin and subcutaneous tissue .
What is the diagnosis code for dry skin?
dry skin (L85. 3)
What is the ICD-10 code for loose skin?
ICD-10 code: L98. 7 Excessive and redundant skin and subcutaneous tissue - gesund.bund.de.
What is the ICD 10 code for psoriasis?
L40.9L40. 9 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes.
What is the ICD 10 code for skin infection?
ICD-10 code: L08. 9 Local infection of skin and subcutaneous tissue, unspecified - gesund.bund.de.
What is the ICD-10 code for edema?
R60.9R60. 9 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes.
What is the meaning of xerosis?
Abnormal dryness of the skinXerosis: Abnormal dryness of the skin, mucous membranes, or conjunctiva (xerophthalmia). There are many causes of xerosis, and treatment depends on the particular cause.
Is xerosis a disease?
What is Xerosis? Xerosis is the medical name for dry skin. It comes from Greek: 'xero' means 'dry' and 'osis' means 'disease' or 'medical disorder'. Xerosis is caused by a lack of moisture in the skin, which may be the result of ageing (senile Xerosis) or due to underlying diseases such as Diabetes.
What is the ICD-10 code for skin irritation?
Irritant contact dermatitis, unspecified cause L24. 9 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes. The 2022 edition of ICD-10-CM L24. 9 became effective on October 1, 2021.
What is a belt tummy tuck?
A belt lipectomy is a type of surgery. It's done to remove the loose skin and fat around your waist or “belt line.” This is also called an abdominal lipectomy, tummy tuck, and panniculectomy. You may have this surgery after you lose a great deal of weight.
What is ICD-10 code for unintentional weight loss?
R63.4ICD-10 code R63. 4 for Abnormal weight loss is a medical classification as listed by WHO under the range - Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified .
What is the FDA approved substance for vocal fold injection?
Calcium hydroxylapatite (CaHA), known by the trade name Radiesse Voice, is currently a FDA approved substance for potentially long-term vocal fold injection. It is comprised of microspheres of CaHA in a carboxymethylcellulose carrier. Rosen, et al, (2009) reported that a recent multi-institutional clinical trial revealed excellent results of 80% improvement at 12 month follow-up. Also, Carol, et al (2010) reported that long term clinical results show that persistent medialization after CaHA injection may be present up to two years or more, with an average duration of 18 months.
What is dermal filler?
dermal filler is a product that is injected or placed into the dermis. There are also subdermal fillers and those placed underneath the dermis in the subcutis. They can be injected into areas with fine lines and wrinkles. Bovine collagen was the first FDA-approved dermal filler in the United States for more than a decade. The FDA approved indication for Zyderm is for the correction of contour deformities of the dermis in non-weight bearing areas. This product was marketed as Zyderm I and was extremely effective for correction of fine lines and shallow scars, with results often lasting three months. Other uses were for rhytides, deeper nasolabial folds and marionette lines but the effect only lasted about two months. There were issues with this product that included allergies to bovine collagen, lack of longer lasting results and disappointing results for its uses. Later Zyderm II and Zyplast were FDA approved with hopes of improving on the Zyderm I issues. Zyplast was indicated by the FDA approval for the correction of contour deficiencies of soft tissue and seemed to work well but some of the same issues existed with frequency of treatments, skin allergy testing and the poor response of deeper rhytides and folds.
How long did the Vega study last?
The Vega Study, from France, was a 96-week open-label, non-comparative, single-center study in 50 HIV patients. Patients had a mean age of 45 years, 84% were Caucasian, and 98% were male. Most patients had four to five injection sessions. The results showed all patients experienced increases in skin thickness in the treatment area in weeks eight to 96 during the study and the changes persisted for up to two years. The results also showed 3% had bruising, 30% had hematoma, and 52% had a nodule at the injection site.
When was Sculptra approved?
Sculptra Aesthetic was FDA approved on July 29, 2009 for correction of nasolabial folds and other facial wrinkles. The approval was based on results from a randomized, comparative, valuator-blinded, parallel group, multicenter study of 233 patients. Patients received Sculptra Aesthetic or an approved human derived collagen for the treatment of their nasolabial fold wrinkles. Sculptra Aesthetic was administered in a single treatment regimen, at three week intervals, for up to four treatment sessions for the correction of shallow to deep dermal grid pattern (cross-hatch) injection technique. The Sculptra Aesthetic patients were followed for an additional 12 months. Treatment effects were maintained up to 25 months after the last treatment session, while the human derived collagen was effective up to three months.
Is dermal filler FDA approved?
The use of dermal fillers for the treatment of vocal cord paralysis is not an FDA approved indication and therefore does not meet TEC criteria. The American Academy of Otolaryngology-Head and Neck Surgery Foundation (AAO-HNSF) published national clinical practice guidelines in September 2009.
Is Blue Cross Blue Shield Alabama a medical necessity?
As a general rule, benefits are payable under Blue Cross and Blue Shield of Alabama health plans only in cases of medical necessity and only if services or supplies are not investigational, provided the customer group contracts have such coverage.

Popular Posts:
- 1. icd 10 code for right otalgia
- 2. icd 10 code for intravenous therapy
- 3. icd 10 code for poor emptying
- 4. icd 10 code for preseptal cellulitis
- 5. icd 9 code for parkinson with dementia
- 6. icd 10 code for r05.9
- 7. icd 10 code for svt right basilic
- 8. icd 10 code for solu medrol injection
- 9. what is the icd 10 code for pre eclampsia
- 10. icd 10 code for scattered hematuria